Nuvaxovid
Meet innovative vaccine technology from Novavax. Novavax Nuvaxovid vaccine regulatory information.
Novavax Nuvaxovid Gets Expanded Conditional Marketing Authorization In Eu For Use As Booster For Adults Aged
The vaccine is stored at 2- 8 Celsius enabling the use of existing vaccine supply and cold chain channels.
. Three large clinical trials showed that Novavax is effective in preventing COVID-19 in. As COVID-19 vaccines are administered across Canada our safety monitoring is. Most side effects are mild to moderate in severity and are gone within a few.
Full results from Nuvaxovids pivotal phase III trial were published in December 2021. Sotrovimab sotrovimab GlaxoSmithKline Inc. The Novavax vaccine will be manufactured in two different facilities.
Nuvaxovid führt häufig zu lokalen und systemischen Impfreaktionen die über wenige Tage anhalten können und ähnlich stark sind wie nach Impfung mit den anderen COVID-19-Impfstoffen. Der Impfstoff darf nicht intravaskulär subkutan oder intradermal injiziert werden. Novavax and SK bioscience File a Post Approval Change Application in South Korea for Nuvaxovid COVID-19 Vaccine as a Booster in Adults Aged 18 and Older Download Show 5 10 25 50 100 per page.
It is also available as a booster at least 6 months after completing the primary course for any of the COVID-19 vaccines available in New Zealand. The addition of the saponin-based Matrix-M adjuvant. Nuvaxovid the COVID-19 vaccine developed by Novavax has today been given regulatory approval by the Medicines and Healthcare products Regulatory Agency MHRA.
The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19 for emergency use on 20 December 2021 and 17 December 2021 respectively. Vous trouverez des renseignements techniques détaillés comme la monographie du produit et le résumé de la décision réglementaire en cliquant sur le lien suivant. Lutilisation du vaccin Nuvaxovid de Novavax contre la COVID-19 a été autorisée au Canada au titre du Règlement sur les aliments et drogues.
Nuvaxovid is the first protein-based COVID-19 vaccine granted authorization from the Medicines and Healthcare products Regulatory Agency MHRA. The Novavax COVID-19 vaccine Nuvaxovid is for people aged 12 and over who wish to have a different COVID-19 vaccine option. Clinical trials showed that the vaccine has around 90 efficacy in adults.
Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing ten doses. The Novavax Nuvaxovid COVID-19 vaccine was authorized for use in Canada under the Food and Drug Regulations. 31102022 - Medicinal products dataset.
It will be offered per the Joint Committee on. The immune response to the vaccine is similar in adolescents compared with adults. Nuvaxovid becomes the fifth COVID.
Die Zulassungsstudien ergaben keine Sicherheitsbedenken hinsichtlich schwerer unerwünschter Wirkungen nach Impfung. The Novavax COVID-19 vaccine sold under the brand names Nuvaxovid and Covovax among others is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations CEPI. Authorized By Interim Order.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. Der Impfstoff darf nicht mit anderen Impfstoffen oder. You can read the full Nuvaxovid Novavax Consumer Medicine Information document on the TGA site for more details click I accept to see the PDF.
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Our biotech company prevents a broad range of infectious diseases through recombinant nanoparticle technology.
The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix-M adjuvant given intramuscularly 21 days apart. The benefits of vaccination with Novavax greatly outweigh the risk of side effects. A primary course is 2 doses 3 weeks apart.
Het Novavax-vaccin is volgens het Europese geneesmiddelenagentschap EMA en de Gezondheidsraad veilig voor mensen van 12 jaar en ouder. Find detailed technical information such as the product monograph and the regulatory decision summary. LONDON dpa-AFX - Novavax Inc.
QUICK TAKE NVX-CoV2373 Covid-19 Vaccine 0230. Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing ten doses. Coronavirus Hintergründe Nachrichten Ratgeber und Antworten auf viele Fragen rund um SARS-CoV-2 und die Viruserkrankung COVID-19.
In Europe the vaccine will be. Die STIKO stellt fest dass die Datenlage. NVAX a vaccine maker focused on serious infectious diseases said on Tuesday that an initial one million doses of Nuvaxovid or NVX-CoV2373 Covid-19 vaccine are.
As part of the Commission Pharmaceutical Strategy for Europe and EU Open Data initiative datasets representing the information available on the Union Register will be regularly published here and on the European Data portal once this project leaves the BETA stage. More than a year after its emergence as a global pandemic severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 infection has been associated. NUVAXOVID SARS-CoV-2 recombinant spike protein Novavax Inc.
Nuvaxovid ist ausschließlich zur intramuskulären Injektion vorzugsweise in den Deltamuskel des Oberarms vorgesehen. PAXLOVID nirmatrelvir ritonavir Pfizer Canada ULC. The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix-M adjuvant given.

Nuvaxovid Fifth Vaccine Against Covid Authorised In Eu Euractiv Com

Novavax Nuvaxovid Covid 19 Vaccine Star Pharmacy
Fda To Authorize Novavax S Covid 19 Vaccine Politico
Ema Chmp Recommends Cma For Novavax S Covid 19 Vaccine As Booster

Novavax Non Mrna Covid Vaccine Gets Cdc Approval Npr
Novavax S Covid 19 Vaccine Moves Closer To Fda Authorization Decision Wsj
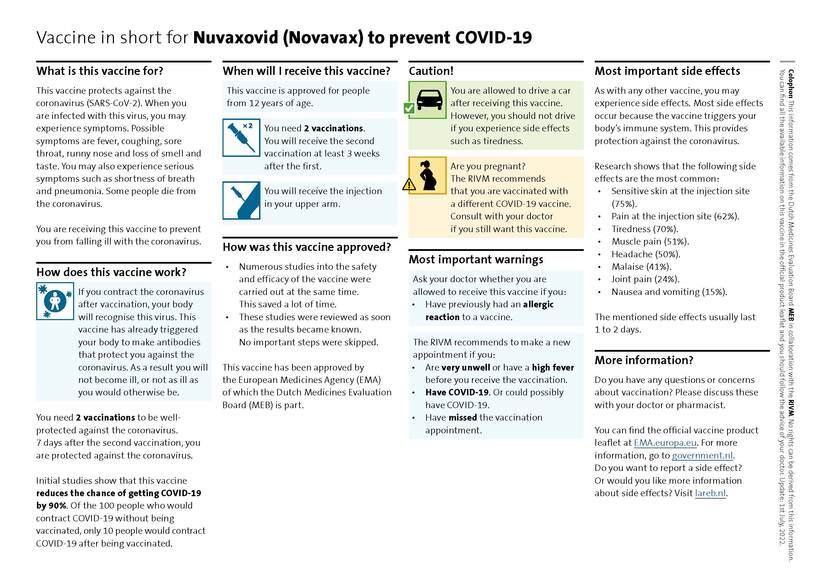
Vaccine In Short For Nuvaxovid Novavax Publication Medicines Evaluation Board

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care
Novavax S Covid Vaccine Rollout In Eu Off To A Slow Start Reuters

My Kingdom For A Booster Shot Hiccups Are Yet Another Vaccine Trial Hassle Medpage Today

Novavax Covid 19 Vaccine To Be Rolled Out In Australia From Next Month

Covid 19 Vaccination About The Nuvaxovid Novavax Vaccine Youtube

Canadian Trademarks Details Nuvaxovid 2163962 Canadian Trademarks Database Intellectual Property And Copyright Canadian Intellectual Property Office Innovation Science And Economic Development Canada
Novavax Nuvaxovid Covid 19 Vaccine Approved In South Korea For Use In Adolescents Aged 12 Through 17 World Pharma Today

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl

Nuvaxovid Novavax Covid 19 Vaccine Course The Immunisation Advisory Centre

Cdc Panel Backs Novavax Covid Vaccine For Adults Medpage Today
